Put electron pairs about each atom such that there are 8 electrons around each atom octet rule with the exception of H which is only surrounded by 2 electrons. Addelectrons for negatively charged ions.

How To Draw Lewis Structures Youtube
Drawing lewis dot structures worksheet.

. Group number for each element of valence electrons. Rules for Drawing Lewis Structure. In writing Lewis structures for covalent compounds you must remember the following.
Draw the Lewis dot structure for CF4. Determine the electron and molecular geometry of the produced molecules. Only C N O P and S rarely Cl will form multiple bonds.
Writing Lewis Dot Structures. Molecule Dot Structure VSEPR shape CF4 Cl20 C2H4 HCN HF PF. Draw bonds as lines between atoms.
If the entire molecule is charged ie. The least electronegative element goes in the center. A polyatomic anion or cation add.
First of all a correct count of all valence electrons is essential. Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula Hydrogen is never the central atom even if it is. 1Count the total number of valence electrons in the molecule make a little table to help you do this.
Draw Lewis structures for atoms ions and simple molecules. Count the total number of valence electrons in the structure Remember Group of Valence electrons 2. Calculate the total number of valence electrons.
Hoeger 1 Determine the total number of valence electrons for ALL atoms. Use Lewis structures as a guide to construct three-dimensional models of small molecules. Given a chemical formula by reference to the Periodic Table we can quickly decide how many valence.
What are the rules for drawing Lewis diagrams for molecules. All valence electrons of the atoms in Lewis structures must be shown. 1 Answer anor277 Mar 26 2018 Just to retire this question.
Drawing Lewis Structures A step-by-step Guide by C. Dont be concerned with which atom gave what. Were going to be working with 6 different molecules or ions.
Recall the rules in drawing a Lewis structure anduse them to drawn the following molecules. Determine the total number of valence electrons. H is NEVER a center atom.
Sometimes its necessary to form double and triple bonds. What this means is that we decide how the atoms are to be bonded. WRITING LEWIS DOT STRUCTURES Lewis structure or formula shows electrondot symbols for the atoms the bonding pairs as lines and the lone pairs that fill each atoms outer level valence shell as pairs of dots.
Molecule Dot Structure VSEPR shape CF4 Cl20 C2H4 HCN HF PF. Unpaired electrons are observed in odd electron molecules such as NO and NO2. Determine the total number of valence electrons.
Here are the five steps to success. 2Build the skeleton of the molecule. The general rules for writing Lewis structures include the following.
Use the octet rule to draw Lewis dot structures for all the stable molecules with the molecular formula mathrmC_3 mathrmH_8 mathrmO. For example the carbon atom in CO 2 in carbon dioxide has four valence electrons and the oxygen atoms have six valence electrons. The SUM TOTAL is what is important.
Draw the initial skeletal structure with atoms connected by single bonds. Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. State the hybridization of the central atom in the stru.
The rule is to get it right Explanation. LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. If the molecule is an ion add or subtract electrons as appropriate.
Make sure that H has only 1 bond and that the other atoms do not exceed their normal number 2 bonds to O 3 bonds to N 4 bonds to C and that no atom in the molecule has more than 4 bonds. Draw a skeletal structure. Ill cover how to properly draw lewis structures of regular molecules and lewis structures of ions.
Subtractelectrons for positively charged ions. Determine the placement of the atoms in your atom using electronegativity values. Determine the total number of valence electrons in the molecule or polyatomic ionSimply.
We will also go over what the octet rule is and all the e. Lewis structures of molecules compounds. General rules for writing LEWIS structures for molecules.
1Using your rules for drawing Lewis Dot Structures draw the following molecules. Determine the total number of valence electrons in the molecule. Generally electrons are paired.
Lewis structures for most molecules can be drawn by following a simple strategy. It should be the most symmetric structure you can draw. Draw the Lewis dot structure for each atom of the molecule to show how many valence electrons are present in each atom of the molecule.
MUST FOLLOW IN ORDER. Scientists often create models to represent either a physical or abstract system or structure that. And recall that for main group elements the valence electrons can be determined from the group number.
CO2 H2O BCl3 PCl3 NH4 and NO2-. Add up the valence electrons of the atoms. H20 h x 0 x 3.
One way to do this is to write the Lewis symbols. Draw ONLY one bond between neighboring atoms. Lewis Dot Structures and Shapes of Molecules What follows is a step-by-step tutorial on how to draw Lewis Dot Structures for molecules and polyatomic ions.
Lewis Structure Of Atoms Worksheet Answers In this worksheet students draw the lewis dot structure for each element molecule and compound. For molecules and polyatomic ions composed of nonmetals. To do this you will need the periodic table.
Each bond counts. 1Using your rules for drawing Lewis Dot Structures draw the following molecules. Steps to Drawing Lewis Dot Structures.
Count the total number of valenceelectrons. Instructions for Drawing Lewis Structures. Choose a central atom.
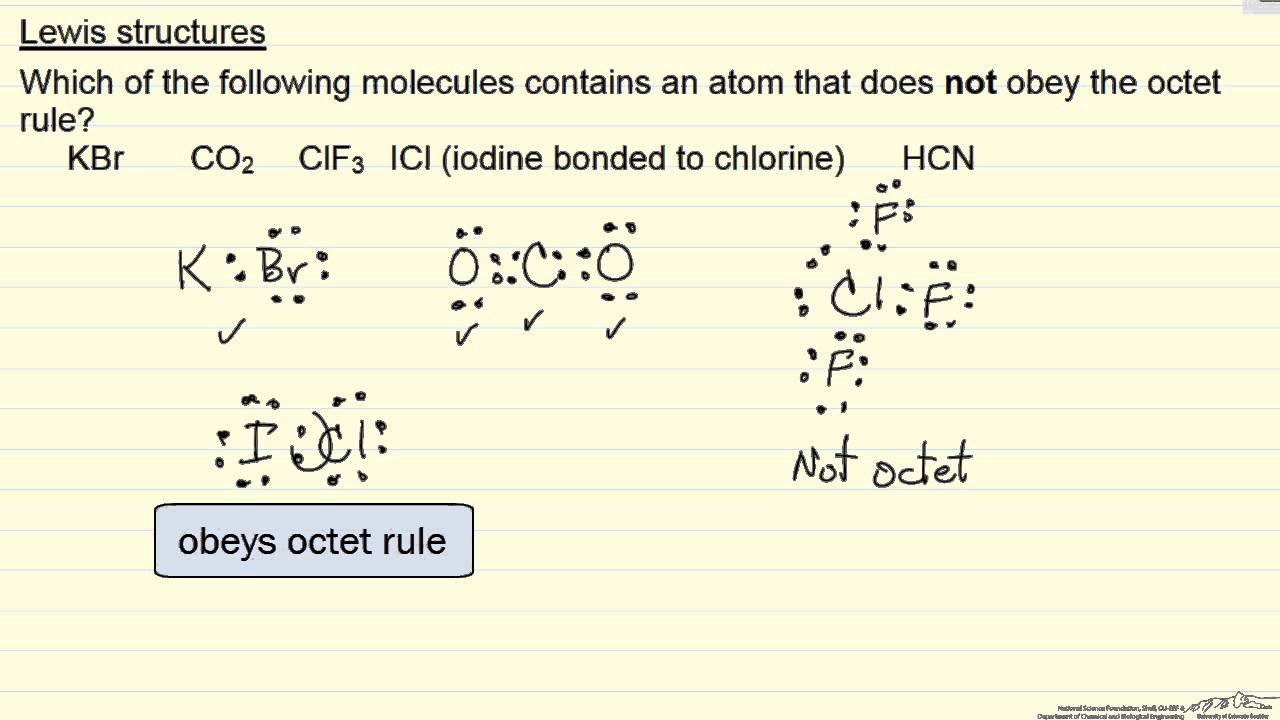
Lewis Structures Octet Rule Example Youtube
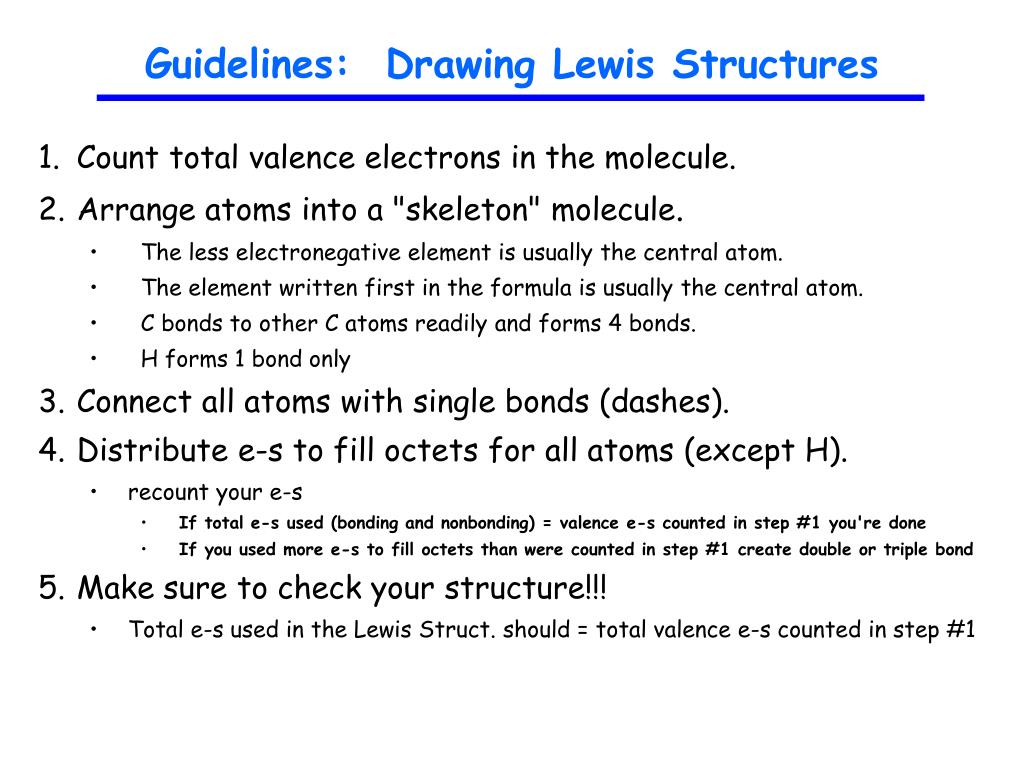
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949
Lewis Electron Dot Structures Help
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)




0 comments
Post a Comment